Chapter Notes: Acids, Bases and Salts
Chapter Notes: Acids, Bases and Salts
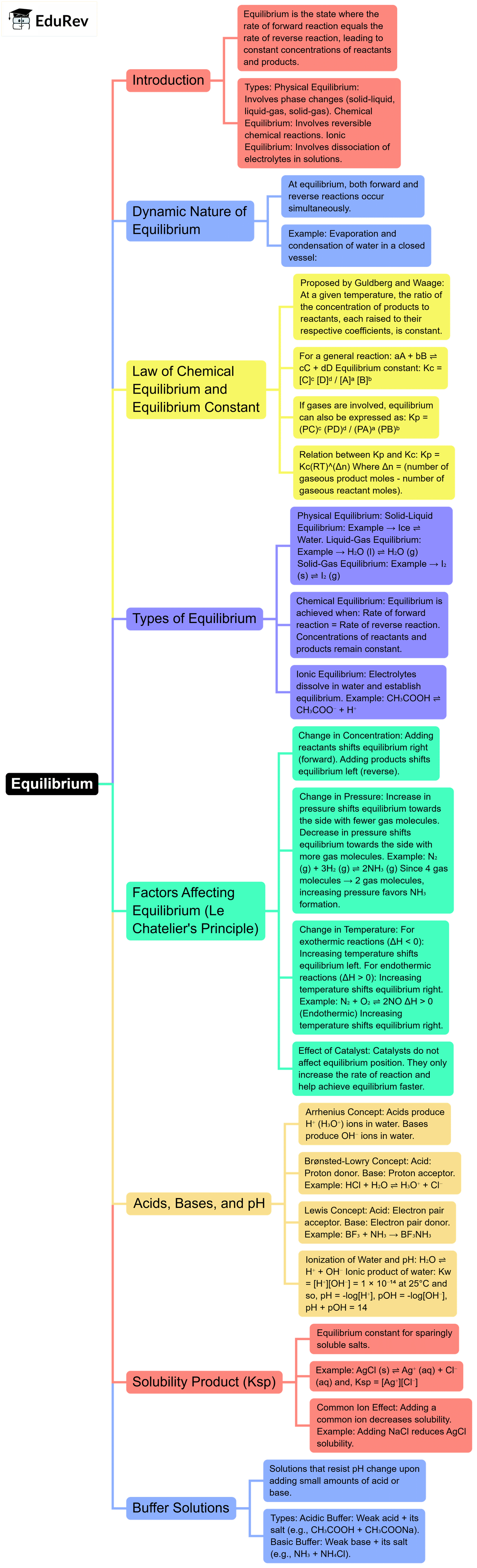
1. Introduction
Acids, bases, and salts are among the most important chemical substances. They are involved in various chemical reactions and have wide applications in industries, biological systems, and daily life.
2. Theories of Acids and Bases
(a) Arrhenius Concept (1884)
-
Acid: A substance that produces H⁺ ions in aqueous solution.
Example: -
Base: A substance that produces OH⁻ ions in aqueous solution.
Example:
Limitations:
-
Valid only for aqueous solutions.
-
Does not explain basicity of substances like NH₃ (which has no OH⁻ group).
(b) Brønsted–Lowry Concept (1923)
-
Acid: Proton donor.
-
Base: Proton acceptor.
Example:
→ NH₃ acts as base, H₂O acts as acid.
Conjugate Acid–Base Pair:
Each acid and base forms a conjugate after donating/accepting a proton.
Example
(c) Lewis Concept (1923)
-
Acid: Electron pair acceptor.
-
Base: Electron pair donor.
Example:
This concept includes acid–base reactions that do not involve protons.
3. Acid–Base Strength
Strong Acids: Completely ionized in water
→ HCl, HNO₃, H₂SO₄
Weak Acids: Partially ionized
→ CH₃COOH, H₂CO₃
Strong Bases: NaOH, KOH
Weak Bases: NH₄OH, Al(OH)₃
4. Ionization of Water
Water is a weak electrolyte that dissociates slightly:
or
Ionic Product of Water:
At 25°C,
5. pH and pOH
pH = –log[H⁺]
→ Lower pH → stronger acid
→ Higher pH → stronger base
pOH = –log[OH⁻]
And,
| Solution Type | pH Range |
|---|---|
| Acidic | < 7 |
| Neutral | = 7 |
| Basic | > 7 |
.png)
Comments
Post a Comment